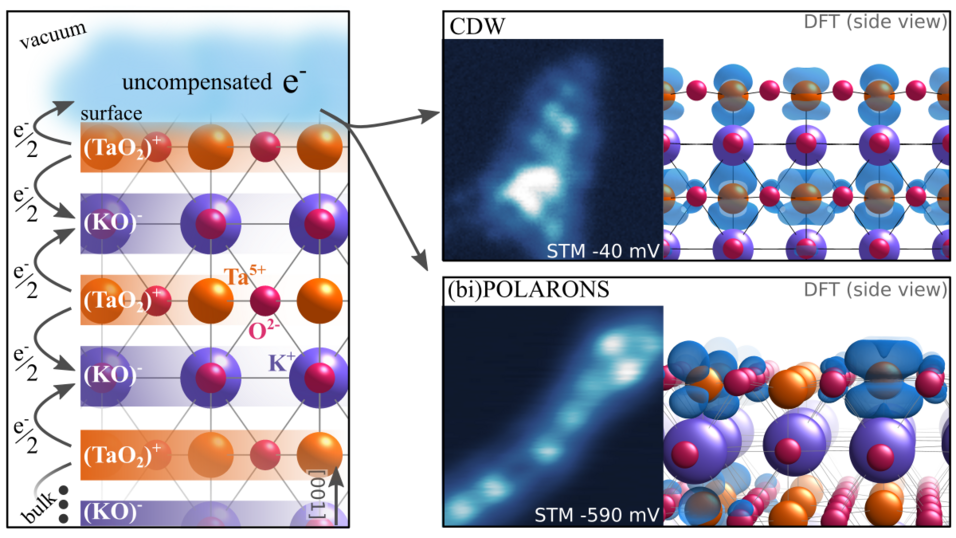
The surfaces of ferroelectrics are particularly interesting in catalysis studies because the material’s polarizability offers one additional parameter that can be tuned (and potentially varied in time) to influence catalytic reactions. Thus, scientists are trying to understand better these materials’ surfaces at the atomic scale. In a new study just published online in Nature Communications, scientists from TACO subprojects P02 and P07 and their international collaborators target the perovskite crystal KTaO3 that forms a prototypical polar surface.