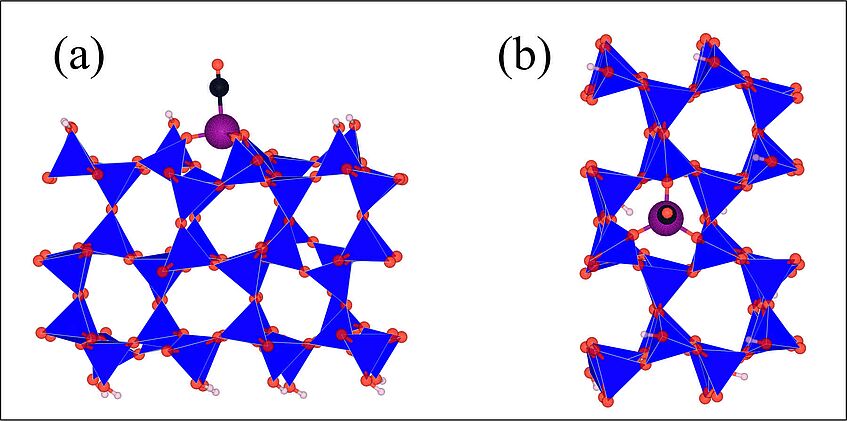
Figure: CO adsorbed on the Cr3+ cation. The Cr3+ cation is an active site on the silica surface produced by the 3H+/Cr3+ ion exchange. (a) side view and (b) top view of the silica layer.
Color code: blue, red, and pink balls are silicon, oxygen and hydrogen atoms. The violet and black balls are chromium and carbon. The blue polyhedra are SiO4 tetrahedra.
Admixtures on solid surfaces acting as catalysts
Industrial processes use catalysts optimized for low cost production. For many of them the optimization is done phenomenologically based on trial and error. Some of the most popular phenomenological catalysts are zeolites or alumina for petrol, and Phillips CrO3/SiO2 or Ziegler-Natta TiCl4/MgCl2 for polyethylene.
In spite of the huge production volumes and large importance of produced materials, many details of the chemical process are still unclear. One reason is that deeper understanding of the reaction scheme is often obscured by lacking knowledge of the configuration of the active site.
Finding the true configuration of the active site on the surface of solid catalysts can be very tricky. Active configurations can be formed by admixtures dispersed on the surface of the support. Low concentrations of admixtures and irregular locations make the detection of active sites using experimental techniques extremely difficult. Alternatively, DFT simulations can be used to study active sites on surfaces of zeolites and silica. Properties of active sites are investigated by DFT simulations of CO adsorption and comparing it with experimental IR data. Sites exhibiting desirable adsorption properties are tested for adsorption of reactants and conversion reactions, such as cracking of long hydrocarbons and synthesis of ethylene to polyethylene.
Contact: Dr. Lubomir Benco
